Our Founders

15+ years in the ENT/Sinus and Allergy field of practice
Otolaryngology, Head and Neck Surgery training at Vanderbilt University
President of Murfreesboro Medical Clinic (100+ physician private practice) 2021-24

10+ years in corporate healthcare as CFO
Economics degree from Harvard and MBA/ JD from Vanderbilt
10+ years working with PE and VC firms in the healthcare industry

The RhineX Solution
Why RhineX? Why now?
For over 20 years sinus surgeons and allergy doctors have been using nasal steroid rinses for their most challenging sinusitis patients to decrease inflammation in the nose. This treatment was so effective they began using it for patients with allergic rhinitis. These steroid rinses, though highly effective, were off label and not FDA approved. RhineX is a bold and innovative treatment strategy to simplify the steroid rinse process for both the patient and the medical provider. Currently patent-pending and under FDA review, RhineX is a breakthrough therapy designed by ENT specialists to make advanced nasal care accessible to everyone.
Every great pharmaceutical story begins with a moment of clarity.
For RhineX, that moment was simple:
People deserve to breathe better.
From that starting point —
- through science,
- through regulatory hurdles,
- through formulation and manufacturing,
- through partnerships,
- through investment
— the mission has never changed.
RhineX is not just a drug.
Not just a sachet.
Not just a regulatory pathway.
It is the culmination of twenty years of clinical reality finally catching up to pharmaceutical innovation.
This is only the beginning.
From Practice to Product

The story is that ENT surgeons first began using budesonide nasal rinses for their most difficult chronic sinusitis patients because nasal steroid sprays were not very effective at delivering steroids to the entire nasal cavity. Over the years, we began to see the utility of using nasal steroid rinses in more and more of our patients including allergic rhinitis patients. At first, nasal steroid rinses were being used on a very small subset of patients, but now we are utilizing them on a wider patient population.
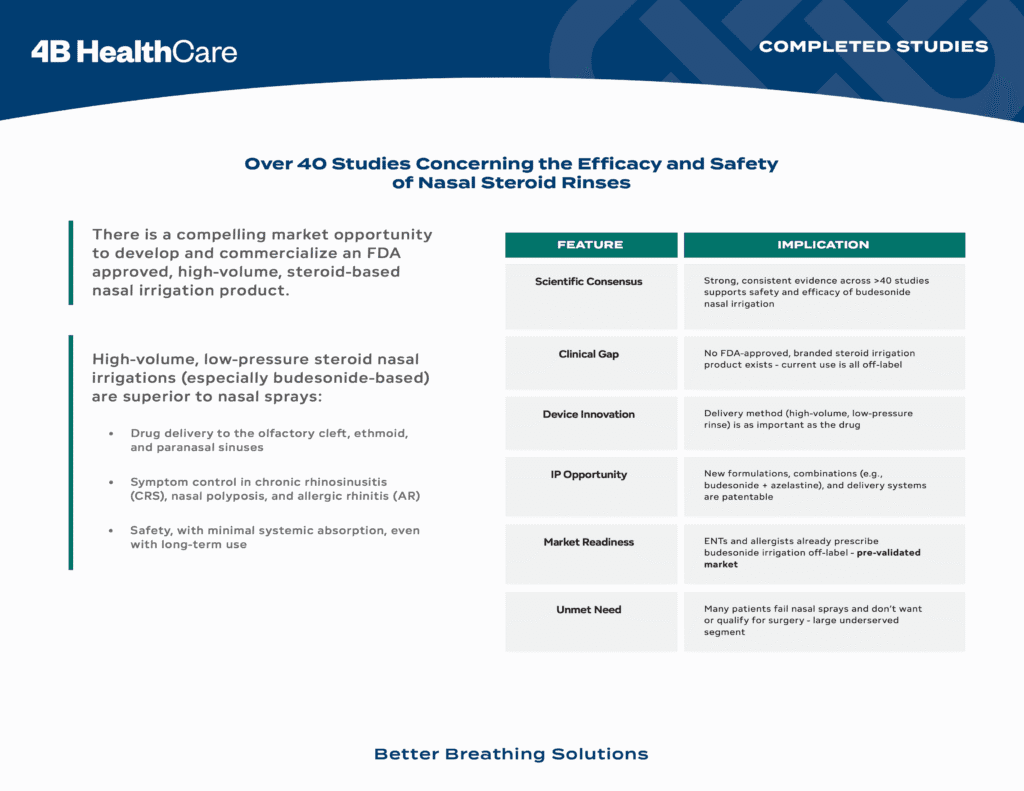
The process of writing a prescription for an off label non FDA approved nasal steroid rinse is cumbersome for the medical provider. The process acquiring and using off label non FDA approved nasal steroid rinse is even more cumbersome for the patient. This is where RhineX steps in. We designed a nasal steroid rinse that is simple for the patient to use and easy for the medical provider to prescribe. We are taking our patent pending nasal steroid rinse through the FDA process and making it available to 60 million allergy sufferers nationwide.
Our Path to Discovery
The Latest in Sinus Care.
Lorem ipsum dolor sit amet, consectetur adipisici elit, sed eiusmod tempor incidunt ut labore et dolore magna aliqua. Excepteur sint obcaecat cupiditat non proident culpa.


